

In the north, electric vehicle users must have encountered the puzzle caused by low temperature weather. When the temperature is too low in winter, battery charging slows down, discharge speeds up, driving mileage is severely shortened, and even face the risk of anchoring roadside in the case of traffic jam. Compared with lithium battery electric vehicles, hydrogen fuel cell electric vehicles are more sensitive to low temperature. Because we know that the only discharge of hydrogen fuel cells is water, which is of great benefit to our users because it is clean and pollution-free. However, for the fuel cells themselves, the water discharged is a potential risk, especially when the temperature drops below zero, the water produced by the fuel cells may freeze. Ice will not only block the reaction gas channel, which will cause the fuel cell to stop working, but also destroy the key components of the fuel cell because of expansion. So if the low temperature starting performance of hydrogen fuel cell is not handled properly, it will not gradually decay like lithium battery electric vehicle, but will probably suddenly fail without warning. At that time, it might not be able to provide enough power for the car to drive home. This potential risk is unacceptable to users. So let's take a look at the key factors of the cold start of fuel cells, the ways to improve the cold start performance, and the challenges facing basic research on this issue.
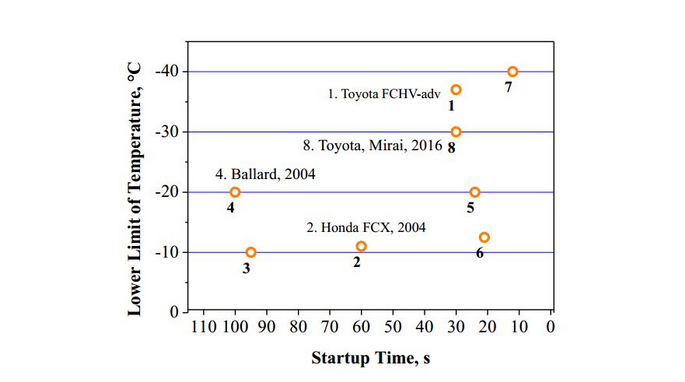
Figure 1. Cold start performance announced by major fuel cell automobile manufacturers
1. Status and Key Factors of Cold Start Performance of Fuel Cell Vehicles
From 2015 to this year, three major global automakers, Toyota, Hyundai and Honda, have released their latest mass-produced fuel cell vehicles to users. In the decades before mass production, major fuel cell manufacturers have conducted in-depth research and Optimization on cold start. As shown in Fig. 1, the cold start temperature of Honda FCX fuel cell vehicle in 2004 was -11 degree C and the starting time was more than 60 seconds. By 2016, the cold start temperature of Toyota Mirai fuel cell vehicle could reach below -30 degree C. In 2005, the U.S. Department of Energy (DOE) set the first cold start target to achieve cold start from – 20 degree C in 2010. The U.S. Department of Energy Fuel Cell Program 2017-2020 requires under the condition of auxiliary cold start at minus 30 degrees Celsius, 50% of rated power can be achieved in 30 seconds..
As we know, there is no obvious difference in the difficulty of oxygen reduction reaction between low temperature and normal temperature. Therefore, the main factor restricting the cold start performance is the various transmission processes within the fuel cell, as shown in Figure 2. The components of proton exchange membrane fuel cell mainly include membrane (PEM), catalytic layer (CL), gas diffusion layer (GDL), microporous layer (MPL) and bipolar plate (BP). Hydrogen and air flow enter the reaction area through the anode and cathode channels respectively. Gas diffusion and convection coexist in porous layers (GDL, MPL and CL). CL consists of a mixture of catalyst particles, ionomers and porous carbon. The electrochemical reaction occurs at the three-phase coexistence site in CL. Fuel cell generates electric current during operation, and water is discharged as reaction product. During the cold start-up period, water changes from one state to another. It can be absorbed by ionomers and turned into membrane water. Because of the temperature below freezing point, some membrane water can be converted into frozen membrane water. Water can also evaporate from ionomers. The resulting vapor permeates through the porous layer and enters the channel. Water vapor may also condense into ice and accumulate in porous layers. In addition, under certain conditions, water can be kept in supercooled liquid state.
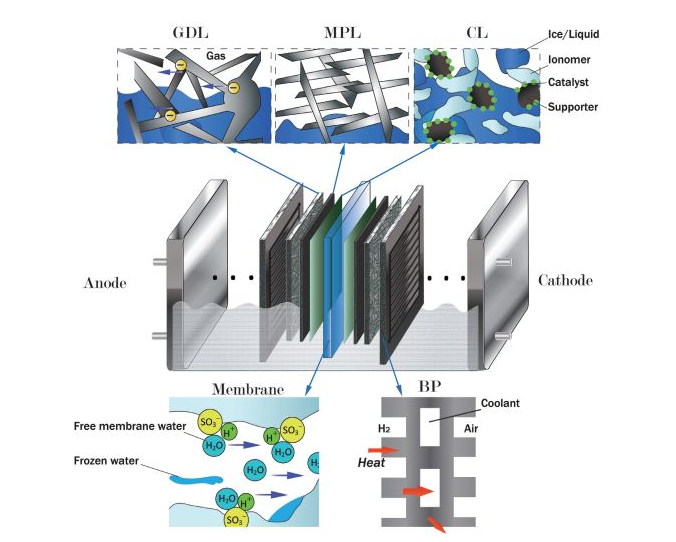
Figure 2. Cold start-up related transfer process of fuel cells
2. Ways to Improve Cold Starting Performance
So, after understanding the principle of fuel cell cold start, another issue we are most concerned about is how to improve the performance of cold start. From theoretical research to mass production of fuel cell vehicles, there are many optimization methods for cold start performance. Here, two technologies applied in fuel cell vehicles are illustrated.
The first method is scavenging. In fact, this is a necessary function of fuel cells for vehicles. The scavenging process can be divided into four stages, as shown in Figure 3. Firstly, it is a two-phase flow problem to sweep the gas flow through the channel and take away the water droplets on the wall of the channel; secondly, the liquid water in the pore of the gas diffusion layer is removed by evaporation and diffusion; thirdly, the liquid water in the pore of the catalyst layer is removed. Finally, the evaporation of membrane water in ionomers. The surface area of ionomers exposed to gases increases with the decrease of liquid water in the voids of the catalyst layer, which further accelerates the removal of membrane water. By changing the scavenging medium gas, the scavenging effect can be further improved. For example, some studies have shown that when helium is used for scavenging, the diffusion coefficient of water vapor is much higher than that when nitrogen is used, thus enhancing the scavenging effect. In order to further improve the cold start performance and to be more convenient for application in automobiles, it is necessary to further improve the scavenging efficiency and shorten the scavenging time in practical application.
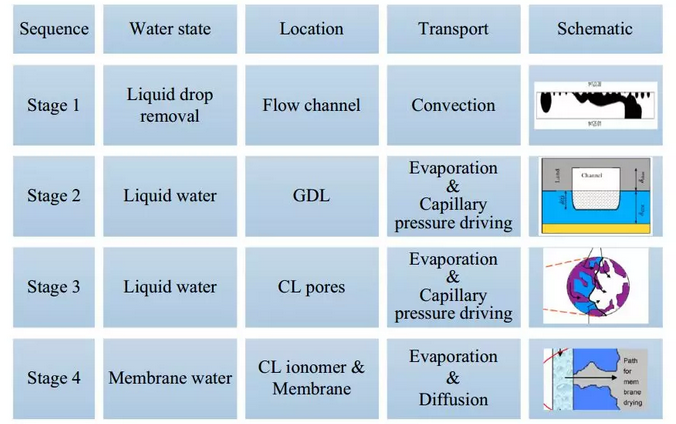
Figure 3. Principle of scavenging effect on cold start
The second method is to use microporous layer (MPL), as shown in Figure 4, hydrophobic and hydrophilic MPL can improve cold start performance. Hydrophilic MPL improves cold start performance by simply expanding the capacity of CL for ice. According to MPL fuel cell, more ice can be accommodated during cold start, and more time can be gained for the success of cold start. The mechanism of hydrophobic MPL improving cold start performance involves supercooled water, which is more likely to remain liquid at higher temperature and smaller droplet size. Therefore, the effect of hydrophobic MPL on cold start-up depends more on the initial temperature. When the temperature is relatively high, for example at – 10 C, the supercooled liquid will stay longer, in which case MPL can force more water into the membrane. Increased conductivity of the membrane can generate more heat, which makes the temperature of the battery increase faster.
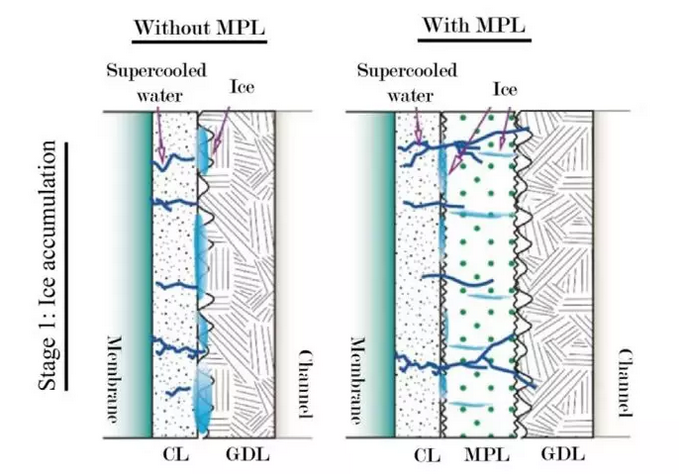
Figure 4. Principle of MPL's influence on cold start
3. Opportunities and Challenges in the Study of Cold Starting of Fuel Cells
The research on cold start of proton exchange membrane fuel cell (PEMFC) is facing three challenges. Firstly, the theory of water phase change is studied, secondly, the modeling and simulation technology, and thirdly, the gap between the performance requirements of cold start and the existing technology. Due to the complex conditions of cold start-up process, the mechanism and properties of icing are still unclear. In the early studies, a large number of cold start models neglected the existence of ice, and these studies mainly focused on heat management. Later, more detailed studies used lumped models or multiphase flow numerical models to describe the processes and effects of ice formation. Although these studies can prove the effect of ice formation on the cold start process, empirical descriptions have proved inadequate. For example, the empirical model can not explain the random variation of cold start performance. The improvement of ice formation theory needs detailed experimental observation, so it is necessary to improve experimental techniques to help observe micro-scale phase transition and transport during cold start. Existing experimental schemes provide some unique techniques to support the microscopic analysis of cold start process, such as CRYO-SEM technology, but because of its static properties, it can not capture the phase transition process. Neutron imaging technology can capture the dynamic process of ice formation, but the scale needs to be further reduced.
For more detailed information and data, please refer to our paper:
Yueqi Luo, Kui Jiao. Cold start of proton exchange membrane fuel cell. Progress in Energy and Combustion Science, Volume 64, January 2018, Pages 29-61.