

In the field of fuel cells, solid oxide fuel cells (SOFC) are another promising energy conversion device in addition to proton exchange membrane fuel cells (PEMFC). SOFC is a high-temperature energy conversion device which directly coverts chemical energy of gasified fuels into heat and electricity. In contrast to PEMFC, SOFC uses a solid oxide electrolyte to conduct oxygen ions from the cathode to the anode, and electrochemical oxidation of oxygen ions with hydrogen or carbon monoxide occurs on the anode side. SOFC can be used from automotive auxiliary power units to fixed power plants from 100W to 2MW, and its theoretical efficiency can exceed 60%. SOFC can also obtain fuel by externally reforming heavier hydrocarbons, such as gasoline, diesel, jet fuel (JP-8) or biomass fuel. The product of the internal and external reforming process is typically a mixture of hydrogen, carbon monoxide, carbon dioxide, steam and methane. The multi-scale schematic of SOFC is as follows:
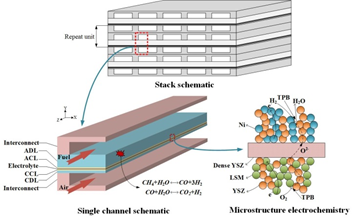
Fig. 1 Multiscale schematic of SOFC
We can also convert and store electrical energy into chemical energy through SOFC's reverse reaction device, Solid Oxide Electrolysis Cell (SOEC). Recently, much interest has been focused on the renewable and eco-friendly power resources due to the increasing energy demand and global environmental concerns. Meanwhile, the meteorological fluctuations of these renewable energies (e.g. solar and wind energy) and the redundant electricity in power grid provide a promising opportunity for energy conversion and storage techniques. Among these techniques, the solid oxide electrolysis cell (SOEC) has aroused much attention for its high efficiency and low emission. Besides, this technology can also offer an approach to directly electrolyze CO2. The co-electrolysis of H2O and CO2 in SOEC generates syngas (H2 and CO mixture), and then the syngas can be further processed to produce other synthetic fuels. For these reasons, the co-electrolysis of SOEC provides a promising pathway for energy storage and transport.
For the anode-supported SOFC, a relatively thick ADL provides sufficient spaces for the internal reforming reactions, and high temperature significantly facilitates the reactions. In this section, the effects of operating pressure on internal reforming reactions inside ADL are investigated with different inlet fuel compositions (30% pre-reformed gas and syngas). Fig. 2 (a) shows the reaction rate of MSRR along the channel length in The MSRR mainly takes place in zones under upstream channel due to the high methane concentration. On account of transverse mass transfer, the MSR rate slightly decreases under interconnect. High operating pressure speeds up the reaction, and this makes MSR concentrate in the entrance zones. In addition, at high operating pressure, methane is mostly converted to carbon monoxide and hydrogen in the region close to the entrance. Thus, at 3 atm, the MSR rate nearly reduces to zero in the midstream region while the rate is zero in the downstream region at 1 atm. Similarly, as shown in Fig. 2 (b), high operating pressure has a remarkable strengthening effect on the WGSR rate at entrance region. But the reaction quickly gets slow as the electrochemical reaction proceeds to consume carbon monoxide along the flow direction. In addition, there is a slightly ascending trend for WGSR rate in downstream region owing to the increased cell temperature.
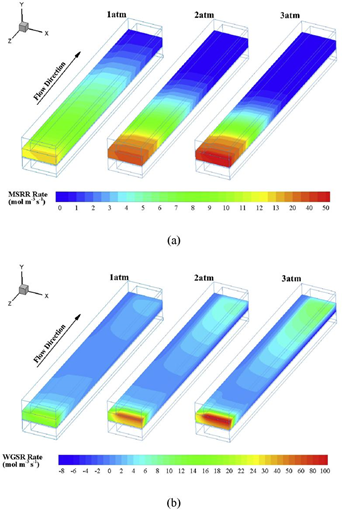
Fig. 2 (a) MSR; (b) WGSR
Generally speaking, for SOEC mode, the WGSR and DIR rates in cathode support layer are affected by the operating pressure. Fig. 4 shows the effect of pressure on WGSR and DIR rates at 1.5 A cm-2 in the middle plane of cathode support layer. The inlet mass flow rate at different pressures are the same, and the inlet gas concentration increases with the increment of pressure. As shown in Fig. 3 (a), nearly no DIR occurs under atmospheric pressure, but the reverse DIR rate increases up to 44 mol m-1 s-1 with the increment of pressure as explained by Le Chatelier's principle. Moreover, this reaction mainly occurs downstream the channel due to the accumulation of hydrogen. As shown in Fig. 3 (b), with the increment of pressure, the reverse WGSR rate increases sharply near the channel inlet and decreases rapidly along the channel, which is caused by the absence of carbon monoxide in cathode feed gas and the consumption of hydrogen along the channel by reverse DIR reaction.
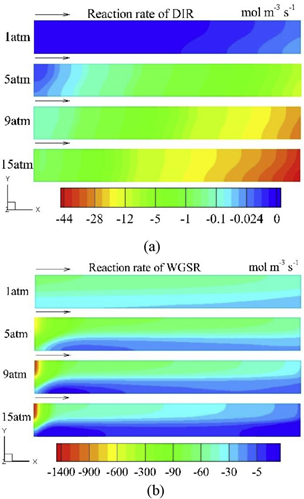
Fig. 3 (a) DIR (MSR); (b) WGSR
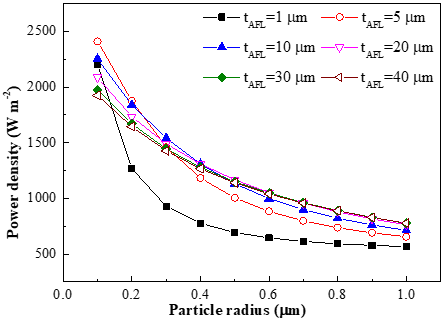
The operation of SOFC always involves complex physical/chemical phenomena such as multi-component diffusion, spontaneous reforming reaction and electrochemical reaction process. Thus we develop an 1D and 2D modeling for solid oxide fuel cell to investigate the internal microstructural effects on cell performance. A comprehensive steady-state model is developed to investigate the effects of electrode structure on the performance of solid oxide fuel cell, considering detailed heat and mass transfer processes, as well as electronic and ionic charge transport. The percolation theory is used to evaluate the effective transport properties in electrode. The uniform and non-uniform distributions of electronic/ionic conducting materials in anode/cathode function layer (AFL/CFL) are comprehensively compared. The effects of function layer thickness and particle sizes are found to be different in anode and cathode. The optimal AFL thickness is increased with the increment of particle sizes. The results show that the optimal AFL thickness ranges from 5 µm to 30 µm with relatively small particle sizes (<0.4 µm), while the cell performance keeps increasing with relatively large particle radius when the AFL grows thicker. Although there exists slight performance drop in condition of non-uniform distribution, the structure reduces ionic-conducting material dosage and fabrication difficulty and it provides another alternative microstructural design which is meaningful to fuel cell optimization.
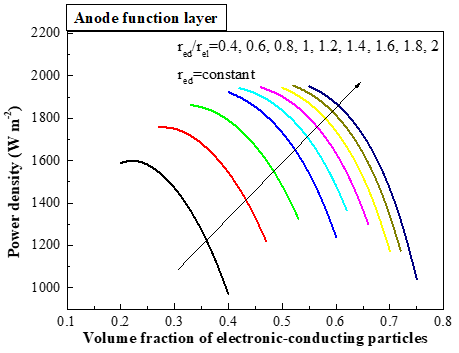
(a) (b)
Fig. 4 the internal microstructural effects on cell performance
We propose an enhanced quasi-two dimensional and non-isothermal model for solid oxide fuel cell (SOFC) parametric simulation and optimization. The dependence of effective properties on microstructural parameters is fully considered in this model. Besides, an elementary effect (EE) approach based on Monte Carlo experiments is adopted to comprehensively evaluate the sensitivity of totally 24 parameters. A two sample Kolmogorov-Smirnov (K-S) test is carried out to evaluate the ability of EE method for robust and accurate sensitivity analysis. The investigation focuses on the important microstructural parameters of the composite anode/cathode function layers (AFL/CFL). With this research, relative volume fractions of conducting materials in the AFL/CFL are the most sensitive factors among all input parameters while particle radius is found to be the least sensitive microstructural parameters. The particle size ratio of electronic particles to ionic particles is found to be much more sensitive than particle size due to its significant effect on effective conductivity. The cathodic electrochemical parameters reflect cell performance more significantly than the anodic ones. To further elucidate the role of input factors, this study provides a principle for parametric sensitivity classification as well. Besides, impacts of current density variation on parametric sensitivity are comprehensively considered. Negative or positive effects of parameters on cell performance and their influencing mechanisms are also discussed. Furthermore, global sensitivity analysis for single parameters at different positions is performed to assess the probability of structural optimization along the channel length. Then a feasible non-uniform distribution method in allusion to function layers is proposed for further improvement of cell performance according to global SA results of single parameters along the channel direction.
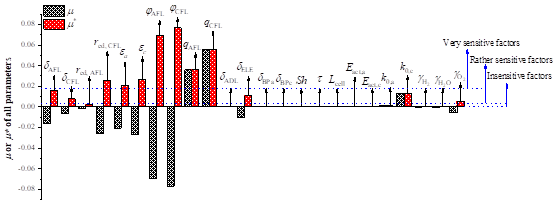
Fig. 5 The dependence of effective properties on microstructural parameters
In general, in the process of environmental friendliness of the coal and energy industry, SOFC technology will also play an important role, and it is widely used as a strategic reserve technology by developed countries in the world. China's SOFC industry started late, and there is still a certain gap between the development of developed countries such as Europe, America and Japan. The commercialization of solid oxide fuel cells in China still has a long way to go.
Reference
(1) Y. Du, Y. Qin, G. Zhang, et al. Modelling of effect of pressure on co-electrolysis of water and carbon dioxide in solid oxide electrolysis cell [J]. International Journal of Hydrogen Energy, 2019, 44(7): 3456-3469.
(2) Y. Wang, R. Zhan, Y. Qin, et al. Three-dimensional modeling of pressure effect on operating characteristics and performance of solid oxide fuel cell [J]. International Journal of Hydrogen Energy, 2018, 43(43): 20059-20076.
(3) C. Wu, Z. Yang, S. Huo, et al. Modeling and optimization of electrode structure design for solid oxide fuel cell [J]. International Journal of Hydrogen Energy, 2018, 43(31): 14648-14664.